Clinician's User Manual Summary of 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple [...]
Prof Mike P Wattjes, MD, Prof Olga Ciccarelli, MD, Daniel S Reich, MD, Prof Brenda Banwell, MD, Prof Nicola de Stefano, MD, Prof Christian [...]
Several organizations have expressed interest and published guidelines or consensus statements emphasizing the need for palliative care for [...]
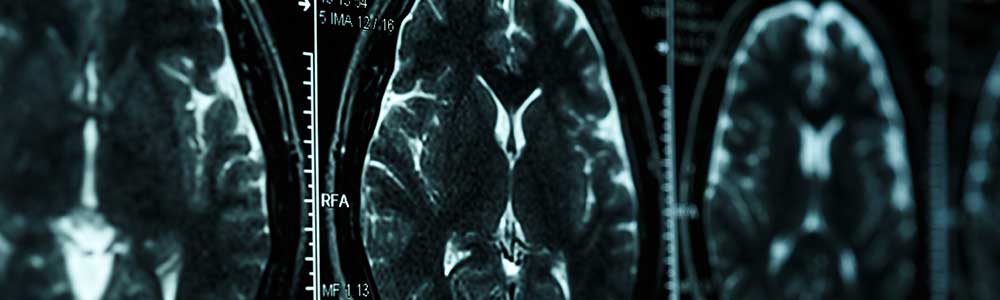
MRI is widely used for the early and accurate diagnosis of multiple sclerosis (MS) and is used increasingly in patient follow-up. [...]
MRI is widely used for the early and accurate diagnosis of multiple sclerosis (MS) and is used increasingly in patient follow-up. [...]
MRI is widely used for the early and accurate diagnosis of multiple sclerosis (MS) and is used increasingly in patient follow-up. [...]
A. Traboulsee, J.H. Simon, L. Stone, E. Fisher, D.E. Jones, A. Malhotra, S.D. Newsome, J. Oh, D.S. Reich, N. Richert, K. Rammohan, O. [...]